Maillard Reaction Of Vitamin C
Once the vitamin C has been oxidized it can react further into a lot of different pathways. One of which is the Maillard reaction. The Maillard reaction causes browning of a lot of food products through a very complex series of reactions.
Besides the Maillard reaction there are a lot of different ways dehydroascorbic acid can react further. But for now, we wont discuss those into great detail, there would be too many. Also, trying to prevent oxidation of ascorbic acid is probably a better way to go than preventing these other reactions. Dehydroascorbic acid is simply less stable and most likely harder to control.
Lesson learned? If you do not want to get scurvy, please do not store your orange juice in a copper pan, in a hot place, with a lot of oxygen in great sunlight. Just squeeze your oranges and drink the same day. Do think about whether you want to squeeze or blend
Vitamin C Dosage In Stressful Patients
In people under stressful conditions either physically or emotionally, vitamin C deficiency is a common incidence. Serum levels of stress indicators were measured in pilots and reported as following 21.1% higher malondialdehyde , 21.7% higher superoxide dismutase , and 25.1% higher total antioxidant capacity . Higher doses of vitamin C are recommended . Among animals, their bodies can produce 5 times of vitamin C when exposed to stressful conditions. Doses of 30200 times greater than the RDA of 90 mg/day are recommended during stress .
Absorption Metabolism And Excretion
From the U.S. National Institutes of Health: “Approximately 70%90% of vitamin C is absorbed at moderate intakes of 30180 mg/day. However, at doses above 1,000 mg/day, absorption falls to less than 50%.” It is transported through the intestine via both glucose-sensitive and glucose-insensitive mechanisms, so the presence of large quantities of sugar in the intestine can slow absorption.
Ascorbic acid is absorbed in the body by both active transport and simple diffusion. Sodium-Dependent Active TransportSodium-Ascorbate Co-Transporters and Hexose transporters are the two transporter proteins required for active absorption. SVCT1 and SVCT2 import the reduced form of ascorbate across plasma membranes.GLUT1 and GLUT3 are glucose transporters, and transfer only the dehydroascorbic acid form of vitamin C. Although dehydroascorbic acid is absorbed in higher rate than ascorbate, the amount of dehydroascorbic acid found in plasma and tissues under normal conditions is low, as cells rapidly reduce dehydroascorbic acid to ascorbate.
Numerous analytical methods have been developed for ascorbic acid detection. For example, vitamin C content of a food sample such as fruit juice can be calculated by measuring the volume of the sample required to decolorize a solution of dichlorophenolindophenol and then calibrating the results by comparison with a known concentration of vitamin C.
Also Check: What Is Vitamin D Good For In Your Body
Side Effects Of Ascorbic Acid
Ascorbic acid is a water-soluble vitamin, and with the dietary excesses that are not absorbed, and the excesses in the blood is rapidly excreted in the urine, it exhibits under these conditions a remarkably low acute toxicity. More than 2 to 3 grams of vitamin C may even cause indigestion, particularly when you take it on an empty stomach. However, taking ascorbic acid in the form of sodium ascorbate or calcium ascorbate may tend to minimize this effect.
Other symptoms that are reported for large doses of vitamin C include nausea, diarrhoea, abdominal cramps. These effects happen due to the osmotic effect of the unabsorbed vitamin C that passes through the intestine. In theory, a higher vitamin C intake may even cause excessive absorption of iron in the body.
Referencesisbn Links Support Nwe Through Referral Fees
- Atkins, P., and L. Jones. 2005. Chemical Principles, 3rd ed. New York: W.H. Freeman. ISBN 071675701X.
- Bánhegyi, G., and J. Mándl. 2001. The hepatic glycogenoreticular system. Pathol Oncol Res 7:107-110. PMID 11458272.
- Combs, G. F. 1998. The Vitamins, Fundamental Aspects in Nutrition and Health, 2nd ed. San Diego, CA: Academic Press. ISBN 0121834921.
- World Health Organization. 2004. Vitamin and mineral requirements in human nutrition, 2nd ed.. World Health Organization. Retrieved February 20, 2007.
Read Also: Does Vitamin D Help Osteoporosis
The Structure And Properties Of Ascorbic Acid
Ascorbic acid is a weak organic acid that appears as a white, crystalline compound. Structurally, it is related to the six-carbon sugarglucose, from which most animals are able to derive the molecule in a four-step process. Like glucose, ascorbic acid is soluble in water.
The ionized form of ascorbic acid is known as ascorbate. The ascorbate ion represents what is called the pharmacophore of vitamin C that is, the structural feature responsible for the molecules biological activity . It is the presence of the ascorbate ion that contributes to vitamin Cs role as a strong reducing agent .
Ascorbate occurs in two forms, both of which are mirror images of the same molecular structure . Vitamin C is specifically the -enantiomer has no physiological significance. L-ascorbate naturally occurs either attached to a hydrogen ion, forming ascorbic acid, or joined to a metal ion, forming a mineral ascorbate.
When -ascorbate carries out its reducing function, it is converted to its oxidized form, L-dehydroascorbate, which can then be converted back to the active form in the body by specialized enzymes and the peptide glutathione.
Effect Of Vitamin E On The Stability Of Vitamin C In Orangejuice
Vitamin E is a fat soluble antioxidant that has four tocopherolsand four tocotrienols. In nature, these four tocopherols and fourcorresponding tocotrienols are designated as alpha-, beta-,gamma- and delta- according to the number and position ofmethyl substituent in chromonal ring . The vitaminE functions as a biological antioxidant by protecting the vitalphospholipids in cellular and subcellular membranes fromperoxidative degeneration. Vitamin E mostly accumulates in body which are liver and pancreas. But unlike vitamins A and D, vitaminE is essentially nontoxic .
Nagymate and Fodor have designed a method to studythe effect of vitamin E on the stability of vitamin C. In this experiment,vitamin E stock solution was prepared by dissolving ?-tocopherol inabsolute ethanol. The orange juice which contained vitamin E andvitamin C was used as sample. The storage temperature of the vialswas 4° C and they were covered with aluminium foil to preventthe effect of sunlight. Besides, two different temperatures wereused to examine the effect of vitamin E at that temperature whichhalf of the samples were stored at 20° C. On the other hand, theadditive effect of these vitamins was also examined but only coolsamples were used for this experiment. Two sampleswere prepared which one contained vitamin E stock solution andvitamin C stock solution while another contained only vitamin Cstock solution. The samples were analysed once a week for fiveweeks .
You May Like: What Can You Take For Vitamin D
Effect Of Ph On The Stability Of Vitamin C
pH is a measure of acidity or basicity of a solution. pH is oneof the primary factor that would affects the stability of vitamin Cin orange juice. Hence, the pH value of the matrix has an influenceon the stability of vitamin C. According to FAO/WHO ExpertConsultation on Human Vitamin and Mineral Requirements,Bangkok, Thailand, 1998, the vitamin C will decay if the pH higherthan 4 . Vitamin C is unstable in neutral and alkaline environments, therefore the higher the pH value andthe longer the exposure, the greater the loss of vitamin C. This isbecause the higher the pH value, the faster the oxidation reaction ofvitamin C and causes the degradation of vitamin C. Besides that, theincrease in pH also related to deterioration of fruit characteristicwhich in this literature review, orange juice is more concerned.Table 7 below shows the pH value of the fruit juice with storagetime .
Table 8: Reaction rate constants for ascorbic acid degradation in the presence of hydrogen peroxide in various fruit juices duringstorage.
Table 9: Natural Orange Juice Ascorbic acid content at different storage times after squeezing.
Analysis of Vitamin C Content in Vitamin C Tablets
Instrument, Materials and Chemicals
The instrument that used in this research is Reflectometer
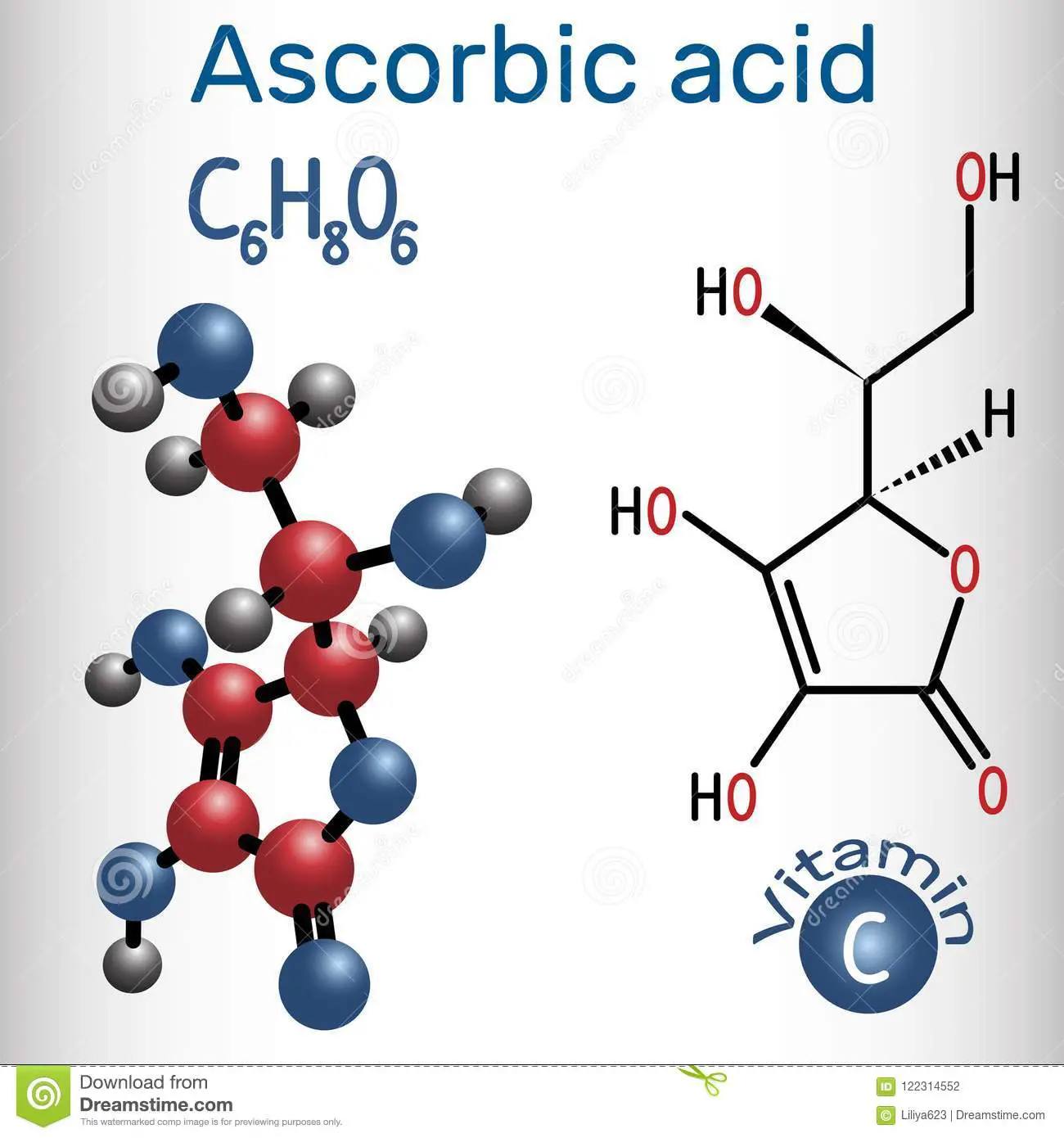
Ascorbic Acid Formula: Structure Molecular Mass Properties
Ascorbic Acid Formula: We all know the importance of Vitamin C in our body, but we should also know its entire chemistry! The chemical name of Vitamin C is Ascorbic Acid, having the chemical formula \ It belongs to the monosaccharide family.
Ascorbic acid is the most heat-labile among all vitamins and is sensitive to heat. Due to this reason, it leaches out from the vegetables containing Ascorbic Acid when immersed in hot water. It acts as an antioxidant that helps to fight bacterial infections and wound healing.
Vitamin C is also water-soluble, so it cannot be produced or stored in the human body. Thus, it must be ingested through our diet. The various sources of Ascorbic Acid or Vitamin C are citrus fruits and some fresh vegetables. Amla is one of its richest sources. In this article, lets learn everything about Ascorbic Acid Formula. Read on to find more.
Recommended Reading: Is There A Vitamin That Helps With Weight Loss
Synthesis Of Ascorbic Acid
Though vitamin C is found in fruits and vegetables, it must be synthesised artificially in laboratories and industries to meet the increasing demand in the pharmaceutical industry. Ascorbic acid is synthesised industrially using a combination of chemical and microbial methods, with D-Glucose as the starting material. This process is known as the Reichstein process.
Chemistry Of Ascorbic Acid
l-Ascorbic acid|
|
| Properties |
|---|
Ascorbic acid is an organic compound with formula C6H8O6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. It is a mild reducing agent.
Ascorbic acid exists as two enantiomers , commonly denoted “l” and “d” . The l isomer is the one most often encountered: it occurs naturally in many foods, and is one form of vitamin C, an essential nutrient for humans and many animals. Deficiency of vitamin C causes scurvy, formerly a major disease of sailors in long sea voyages. It is used as a food additive and a dietary supplement for its antioxidant properties. The “d” form can be made via chemical synthesis but has no significant biological role.
Don’t Miss: Can Vitamin B Help With Weight Loss
Side Effects Of Vitamin C Or Ascorbic Acid
There is an estimated quantity of Vitamin C intake in our body. If it is taken in excess amounts, it leads to acute toxicity.
Study About Sulfurous Acid Formula
Uses Of Vitamin C Or Ascorbic Acid
Vitamin C is necessary for our body as it performs various crucial functions, and its deficiency causes many diseases. Lets have a detailed discussion on its uses:
Recommended Reading: Does Vitamin C Dry Out Skin
Stability Of Vitamin C In Orange Juic
Vitamin C is very susceptible to chemical and enzymaticoxidation during the processing, storage, and cooking of food.The catalyzed oxidation pathway of vitamin C degradation is themost important reaction pathway for the loss of vitamin C in foods.Therefore, vitamin C of orange juice is readily oxidized and lostduring staying of the juice . On the other hand, thereare several factors that will also affect the stability of vitamin C inorange juice. The factors are such as the effect of vitamin E, pH,and parameters which include air, heat, water as well as prolongedstorage and overcooking .
According to Ball, a meta-oxygen-ascorbate complex is formedin the presence of molecular oxygen and trace amounts of transitionmetal which particularly are copper and iron . This complexcontains a resonance form of a diradical that rapidly decomposeto give the ascorbate radical anion, the original metal ion, andhydrogen peroxide. This radical anion will in turn reacts withthe oxygen to give dehydroascorbic acid . For anaerobicpathway of vitamin C which occurs in the absence of free oxygen,the degradation is caused by the formation of diketogulconic acid.As the rate of degradation is maximum at pH 3 to pH 4, thereforethis pathway is mostly responsible for anaerobic loss of vitamin C incanned grapefruit and orange juices .
Some Details About Vitamin C Chemical Formula Chemistry
Possible side effects: Syncope · Dizziness
May treat: Upper Respiratory Tract Infection
People also search for: Ascorbic Acid · Vitamin A · Vitamin E · Vitamin · Vitamin D · B vitamins · Vitamin K · Thiamine · Sodium ascorbate · Riboflavin
PubChem CID: 54670067 · as salt:23667548
UNII: PQ6CK8PD0R · as salt:S033EH8359
Recommended Reading: What Vitamins Should A 20 Year Old Woman Take
Vitamin C Dosage In Pregnant Patients
In females, the hormonal changes result in increased oxidative stress. Lower vitamin C levels are usually detected in pregnant women due to several factors obesity and iron intake which could result in low birth weight .
Up till now, there is no evidence supporting harmful effect on using ascorbic acid injection for pregnant females. Caution should be taken when administrating injection in nursing women.
Uses Of Ascorbic Acid
- It is used in the treatment of scurvy
- It is used in the formation of collagen fibres in connective tissue, fibrous tissue, bones, and teeth
- It fights against bacterial infections
- It functions in detoxifying reactions
- It is used in preventing the transfer of HIV from mothers to babies
- It is used in the treatment of acne and gum infection
- It is used to prevent gallbladder disease
- It is used in the treatment of stomach ulcers caused by Helicobacter pylori
Read Also: Does Vitamin C Help Psoriasis
Chemical Formula And Structure Of Vitamin C
The chemical formula of Vitamin C or Ascorbic Acid is \ It is an organic compound also known as Hexuronic acid, i.e., it is a uronic acid derived from a hexose. Ascorbic acid exists as two enantiomers that are denoted as \ and \ types.
Vitamin C is purely the L-enantiomer of Ascorbic Acid. In contrast, the opposite D-enantiomer has no physiological significance. Both \ and \ forms differ in the orientation at the last chiral centre having the same \ molecular structure. Intramolecular hydrogen bonding is also present in this compound. The word Ascorbic is derived from anti-scurvy because its deficiency causes a disease known as scurvy.
Vitamin C Is A Beneficial Antioxidant
Along with vitamins A and E, and a group of related compounds called coenzyme Q, vitamin C also acts as a general antioxidant. Antioxidants work by making themselves available for energetically favorable oxidation. Free radicals, which may be produced by the body or generated by environmental conditions, such as exposure to ultraviolet light and tobacco smoke, contain an unpaired electron and thus are highly reactive. They may, for example, oxidize the lipid molecules that make up cell membranes and other vital tissues, altering their function. Antioxidants like ascorbate react readily with these free radicals before they can react with other compounds in the body. Radicals oxidize ascorbate first to monodehydroascorbate and then to dehydroascorbate. The radicals are reduced to water, while the oxidized forms of ascorbate are relatively stable and unreactive.
Also Check: How To Make Vitamin C
Faqs Based On Ascorbic Acid
Question 1: How to produce ascorbic acid?
Answer:
Utilizing fermented maize syrup imported from Chinawhich may or may not be genetically altered synthetic vitamin C is produced. After that, solvents such acetone, sulfuric acid, or sodium hydroxide are used to treat the corn syrup in order to extract the ascorbic acid.
Question 2: Does ascorbic acid increase the acidity of the stomach?
Answer:
Due to its high acidity, ascorbic acid can have severe adverse effects on the digestive system when consumed on an empty stomach . Ascorbic acid has a low pH, thus calcium ascorbate was developed to lessen the adverse affect on the epigastric region.
Question 3: Which foods contain a lot of ascorbic acids?
Answer:
Broccoli, cantaloupe, cauliflower, kale, kiwi, orange juice, papaya, red, green, and yellow pepper, sweet potatoes, strawberries, and tomatoes are among the foods high in vitamin C.
Question 4: Ascorbic acid is a potent reducing agent, so why?
Answer:
Ascorbate acts as an electron donor, which explains for all of its known physiological and biochemical functions. AscH is an effective antioxidant and reducing agent because of its ability to donate one or two electrons.
Question 5: Why is ascorbic acid soluble in water?
Answer:
Ascorbic acid is a cyclic polar molecule, and its solubility rises in greater polarity solvent systems. When miscible cosolvents are added to the original solvent , the overall polarity is effectively reduced.
arrow_drop_up